TRANSFORMATION OF SUBSTRATE TO PRODUCT
● We have already understood the idea of an `color{brown}("‘active site’.")`
● The chemical or `color{violet}("metabolic conversion")` refers to a reaction.
● The chemical which is converted into a product is called a `color{brown}("‘substrate’.")`
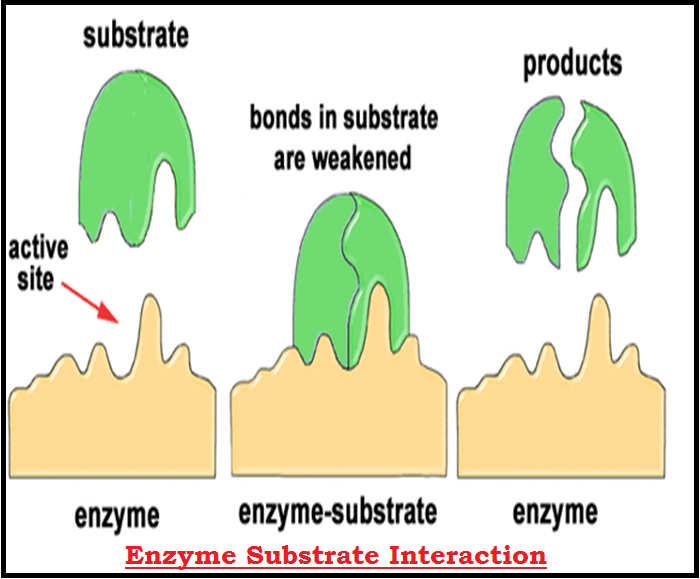
● Hence `color{violet}("enzymes,")` i.e. proteins with `color{violet}("three dimensional structures")` including an `color{violet}("‘active site’, convert")` a substrate (S) into a product (P).
● Symbolically, this can be depicted as: `color{brown}("S →P")`
● It is now understood that the substrate `‘S’` has to bind the enzyme at its `color{violet}("‘active site’")` within a given `color{brown}("cleft or pocket. ")`
● The substrate has to `color{brown}("diffuse")` towards the `color{violet}("‘active site’.")`
● There is thus, an obligatory formation of an `color{brown}("‘ES’ complex. ")`
● `color{violet}("E stands")` for enzyme.
● This complex formation is a `color{violet}("transient phenomenon. ")`
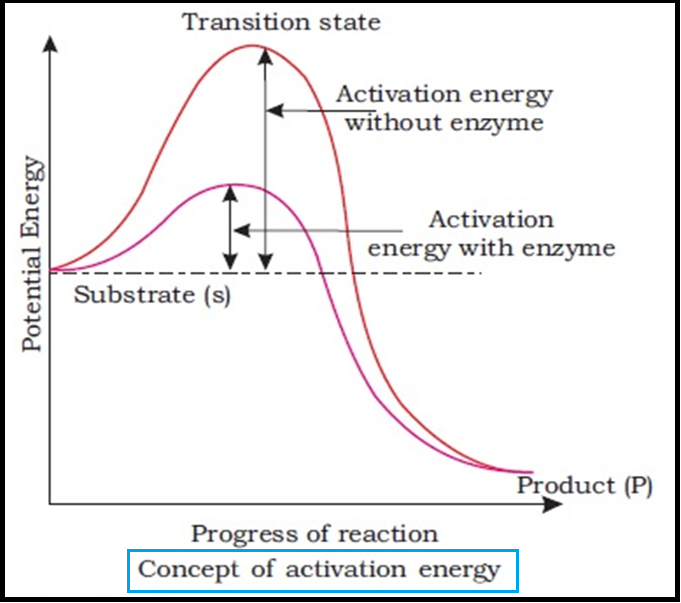
● During the state where substrate is bound to the `color{violet}("enzyme active site,")` a new structure of the substrate called `color{brown}("transition state structure")` is formed.
● Very soon, after the expected `color{brown}("bond breaking/making")` is completed, the product is released from the
`color{violet}("active site.")`
● In other words, the `color{violet}("structure of substrate")` gets transformed into the structure of `color{violet}("product(s).")`
● The chemical or `color{violet}("metabolic conversion")` refers to a reaction.
● The chemical which is converted into a product is called a `color{brown}("‘substrate’.")`
● Hence `color{violet}("enzymes,")` i.e. proteins with `color{violet}("three dimensional structures")` including an `color{violet}("‘active site’, convert")` a substrate (S) into a product (P).
● Symbolically, this can be depicted as: `color{brown}("S →P")`
● It is now understood that the substrate `‘S’` has to bind the enzyme at its `color{violet}("‘active site’")` within a given `color{brown}("cleft or pocket. ")`
● The substrate has to `color{brown}("diffuse")` towards the `color{violet}("‘active site’.")`
● There is thus, an obligatory formation of an `color{brown}("‘ES’ complex. ")`
● `color{violet}("E stands")` for enzyme.
● This complex formation is a `color{violet}("transient phenomenon. ")`
● During the state where substrate is bound to the `color{violet}("enzyme active site,")` a new structure of the substrate called `color{brown}("transition state structure")` is formed.
● Very soon, after the expected `color{brown}("bond breaking/making")` is completed, the product is released from the
`color{violet}("active site.")`
● In other words, the `color{violet}("structure of substrate")` gets transformed into the structure of `color{violet}("product(s).")`